Q 3201734628
 Explain alkyl halide and aryl halide with example
Explain alkyl halide and aryl halide with example


Solution:
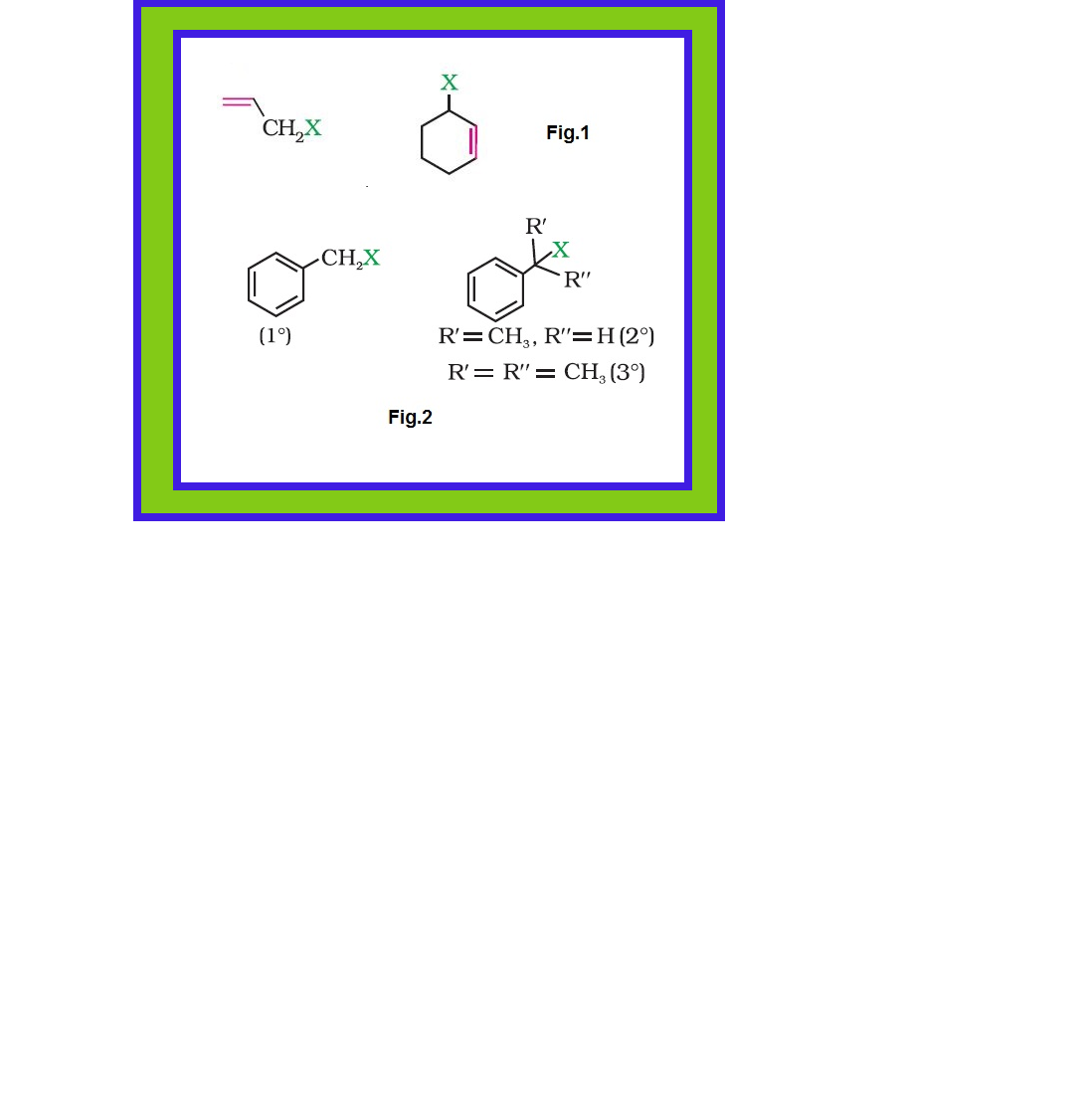
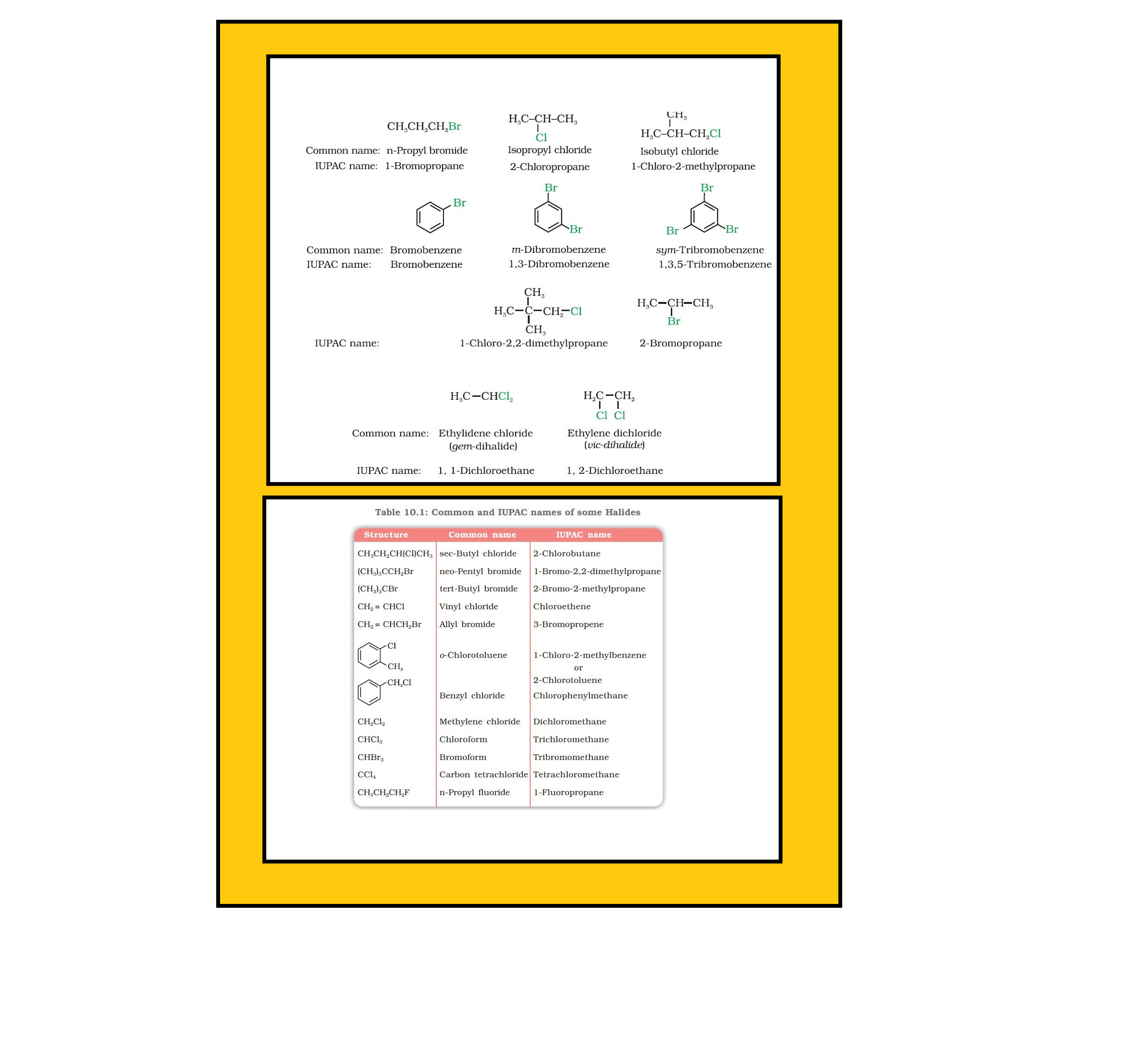
The replacement of hydrogen atom(s) in a hydrocarbon, aliphatic or aromatic, by halogen atom(s) results in the formation of alkyl halide (haloalkane) and aryl halide (haloarene), respectively.
`=>` Haloalkanes contain halogen atom(s) attached to the `sp^3` hybridised carbon atom of an alkyl group.
`=>` Haloarenes contain halogen atom(s) attached to `sp^2` hybridised carbon atom(s) of an aryl group.

The replacement of hydrogen atom(s) in a hydrocarbon, aliphatic or aromatic, by halogen atom(s) results in the formation of alkyl halide (haloalkane) and aryl halide (haloarene), respectively.
`=>` Haloalkanes contain halogen atom(s) attached to the `sp^3` hybridised carbon atom of an alkyl group.
`=>` Haloarenes contain halogen atom(s) attached to `sp^2` hybridised carbon atom(s) of an aryl group.